Custom Market Research Boosts Medical Device Industry Growth
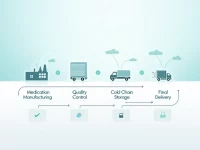
Medical device companies face numerous challenges. Customized research provides insights into market dynamics, competitive landscape, and regulatory environment, helping businesses make informed decisions, mitigate risks, and capitalize on opportunities. This tailored approach allows companies to gain a deeper understanding of the market, identify unmet needs, and develop effective strategies for success. By leveraging comprehensive data and expert analysis, medical device companies can navigate the complexities of the industry and achieve sustainable growth.